March 25, 2025 | Featured
LONDON and BRIDGEWATER, N.J. / ACCESSWIRE / March 25, 2025 – Intract Pharma and Tharimmune, Inc. (Nasdaq: THAR), a clinical-stage biotechnology company focused on immunology and inflammation, today announced positive preclinical results for its novel oral antibody, TH023. In a murine model, a proprietary protease enzyme stabilization platform demonstrated successful delivery of infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, in serum with concentrations detected being significantly higher than the standard serum trough concentration needed for antibody efficacy in immunology indications via injection (~3-5µg/ml)1. These findings represent a significant step towards developing a more convenient and potentially patient-preferred alternative to currently available infliximab treatments, which are administered via intravenous infusion or subcutaneous injection.
Key findings of the preclinical evaluation includes demonstrating enzymatic protection of infliximab against human colon enzymes ex vivo using fresh fecal samples from healthy subjects utilizing the SoteriaTM platform, a proprietary formulation of natural amino acids (data not shown).
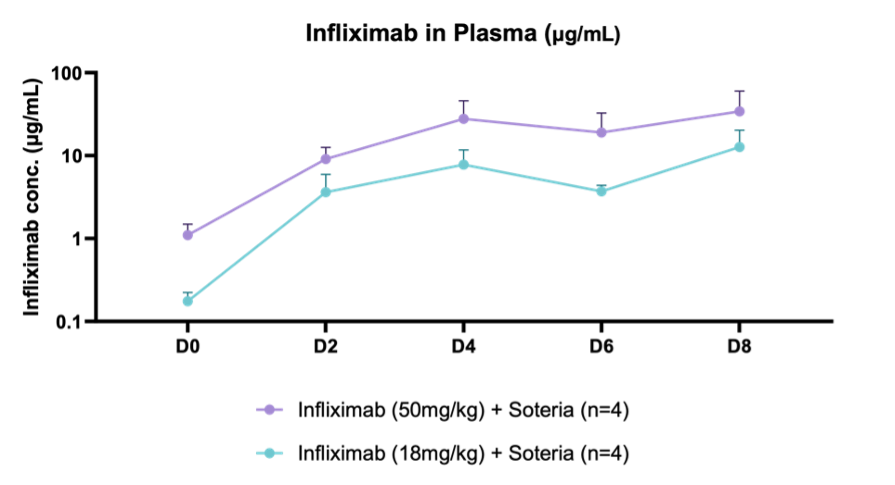
Furthermore, successful delivery of TH023 in-vivo into both local colonic tissue and systemic circulation was shown following intra-duodenal QD dosing for 1 week in a healthy mouse model at two doses of infliximab. This data shows the potential of the delivery platform to allow for both local delivery of the antibody precisely in the large intestine tissue through enzymatic stabilization, as well as systemic circulation, which is an ideal PK profile for targeting local gastrointestinal (GI) diseases such as IBD as well as systemic inflammatory diseases.
The mechanism by which the antibody transcytosis occurs in the GI tract was shown to be a combination of passive and neonatal FcRn mediated active transport, a receptor that’s highly expressed in the distal intestinal epithelial cells.
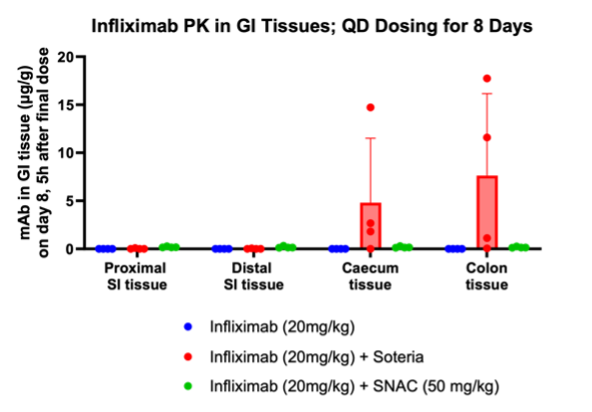
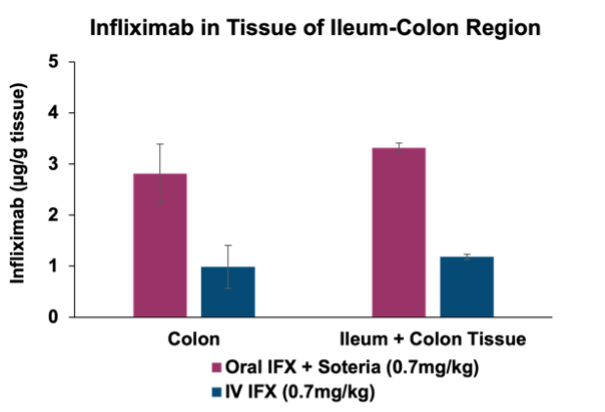
Furthermore, the study demonstrated that tissue penetration of infliximab in combination with the enzyme stabilization platform was superior to a traditional permeation enhancer sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate (SNAC) which has been used to enhance the absorption of GLP-1 peptides such as semaglutide. Utilization of SNAC to protect infliximab from enzymatic degradation or permeation enhancement did not result in tissue or serum concentrations suggesting standard off-the-shelf oral peptide delivery technologies do not work for oral delivery of monoclonal antibodies. We tested two other standard permeation enhancer technologies (sodium caprate and labrasol) which gave the same result (data not shown).
The Company announced last year through a partnership with Intract Pharma an exclusive license to INT-023 (now TH023), an oral anti-tumor necrosis factor alpha monoclonal antibody, infliximab. Tharimmune licensed global development and commercialization rights (outside of South Korea) to Intract Pharma’s Soteria® and Phloral® delivery platform along with an existing supply agreement for infliximab to be used in the oral product development program. Traditionally administered through intravenous infusions, oral delivery of antibodies is challenging due to the complexity of navigating such large molecules through the gastrointestinal tract. An oral route of administration holds potential to improve patient compliance and quality of life, while also reducing the burden on the healthcare system associated with long-term intravenous therapy.
“We are extremely encouraged by these results, which validate the potential of our partnership with Intract and the platform to deliver complex biologic molecules like infliximab orally,” said Randy Milby, CEO of Tharimmune. This represents a potential major milestone in our mission to develop more patient-friendly and accessible treatment options for chronic inflammatory diseases, addressing a multi-billion dollar market. While these are early findings, they provide a strong foundation for further development and eventual clinical trials.”
“These positive preclinical results are an important milestone as we work towards taking this highly validated anti-TNFα antibody in a first-in-class oral pill form using our delivery platform in severe autoimmune indications where patients currently only have injectable options”
Vipul Yadav, CEO of Intract
Through the Company’s existing partnership with Intract the data announced today enables for the targeted delivery of antibody therapeutics directly to the colon or small intestine. By leveraging Intract’s platform, Tharimmune aims to enhance the effectiveness of TNF-α inhibitors such as infliximab through precision delivery that maximizes proteolytic stabilization and tissue permeation. This novel approach offers significant potential for directly addressing inflammatory conditions within the gastrointestinal tract, including inflammatory bowel disease, as well as systemic inflammatory disorders where TNF-α plays a critical role in disease progression. Tharimmune plans to optimize the formulation and dosing regimen and prepare to conduct a first-in-human clinical trial with TH023 in the next 12 months.
Infliximab, a TNF-alpha inhibitor, is a widely used biologic for the treatment of several chronic inflammatory diseases, including Crohn’s disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. Globally, infliximab (including biosimilars) generated approximately $6.3 billion in sales in 2022, demonstrating the significant market for this therapy. However, current administration routes require frequent visits to healthcare facilities, which can be burdensome for patients and contribute to significant healthcare costs. Experts suggest the market for infliximab could rise to 9 billion USD within 10 years. An oral formulation of infliximab has the potential to improve patient convenience and compliance and eliminating the need for injections or infusions which could significantly improve the patient experience and potentially lead to better treatment adherence. Oral administration could reduce the need for clinic visits and specialized nursing care, potentially lowering overall treatment costs.
1Bortlik et al., J Crohns Colitis. 2013 Oct;7(9):736-43
Intract is a biopharmaceutical company developing disruptive oral antibody delivery solutions to significantly improve the efficacy and safety of emerging and established protein therapeutics, as well as improve patient experience and outcomes in inflammation and immunology indications. Its platform leveragesthe advantage of precision targeting large proteins and antibodies to the colon, while also protecting the biologics from enzymatic breakdown, allowing tissue/systemic uptake to create next-generation oral antibody medicines. For more information, please visitwww.intractpharma.com
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune’s future Phase 2 trial, Tharimmune’s strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this release. Subsequent events and developments may cause the Company’s views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this release.
Dr Vipul Yadav
CEO
Tirth T. Patel
212-201-6614
Copyright © 2018 Intract Pharma
A site by: Web Design London